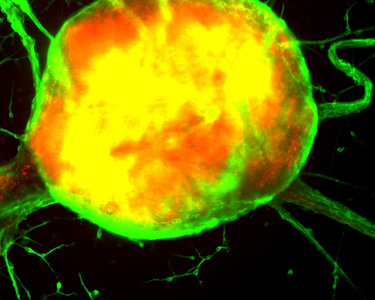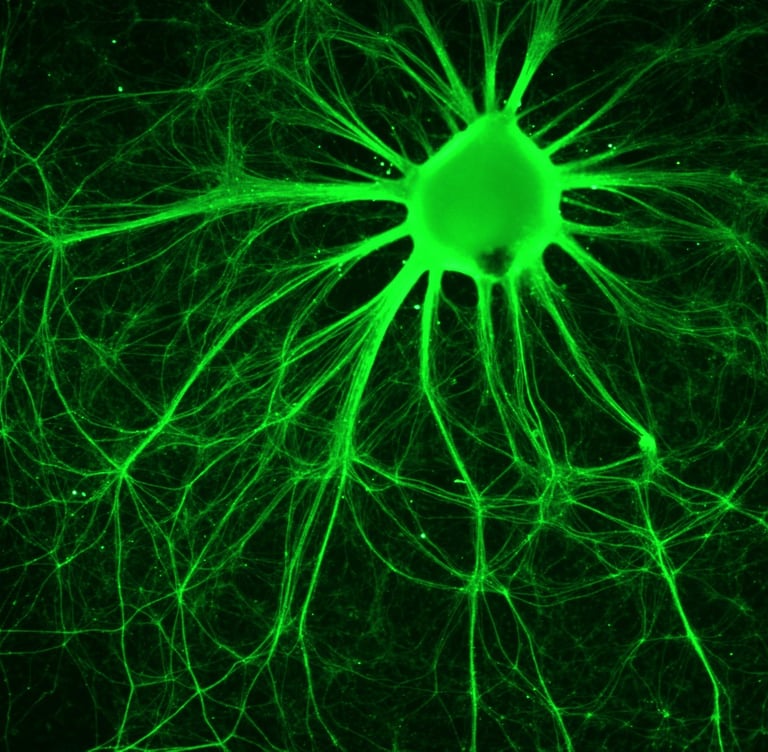
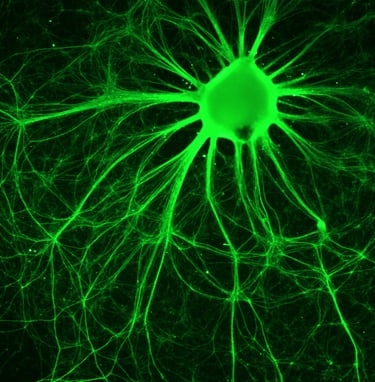
Xcelthera
Impacting the Future of Medicine by Developing Human Embryonic Stem Cell-Based Regenerative Medicine Advanced Therapy (RMAT)
Founded to leverage human embryonic stem cell (hESC)-based regenerative medicine development and manufacturing innovations to provide the next generation of cell-based therapeutic solutions for the unmet medical needs of many major health problems, the Company is a major innovator in the stem cell research and regenerative medicine market, and uniquely holds the key breakthrough technologies/patents/IP/rights for the major bottlenecks in the regenerative medicine market, including large-scale production of high quality clinical-grade pluripotent hESC lines and their functional human neuronal and heart cell therapy products for commercial and therapeutic uses.
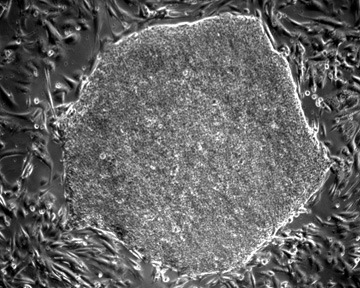
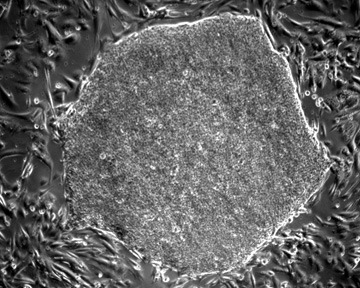
Solving Unmet Medical Needs
Heart and neurological disorders, including heart disease and failure, stroke, Parkinson’s disease (PD), Alzheimer disease (AD), spinal cord injury (SCI), traumatic brain injury (TBI), amyotrophic lateral sclerosis (ALS), spinal muscular atrophy (SMA), are major health problems with estimated costs over $2 trillions annually world-wide. Those devastating and life-threatening diseases are leading causes of death or permanent disability, but there is no effective treatment or drug that can restore the damaged or lost heart or neurological tissues and functions. The limit capacity of cardiomyocytes (the mature contracting heart muscle cells) of the heart as well as neuron circuitries of the brain/spinal-cord for self-repair constitutes a significant challenge to traditional medicine for tissue and function restoration in seeking cures for those serious diseases and conditions. To date, the need to restore vital tissue and function for a wide range of neurological and heart diseases remains a daunting challenge to the conventional mode of drugs and treatments. Although cell-based therapy represents a promising therapeutic approach closest to provide a cure for those diseases, the cell therapy products currently in clinical trials or on the market for heart and neurological indications, if any, have not demonstrated production at scale as well as any regenerative potential for heart muscle or neuron circuitry repair.
The successful derivation of human embryonic stem cell (hESC) lines from the in vitro fertilization (IVF) leftover embryos is considered as one of the major breakthroughs of the 20th century life sciences. Pluripotent hESC can maintain long-term, stable growth and differentiate into clinically-relevant lineages, providing an inexhaustible source of replacement cells for human tissue and function restoration. It is public consensus that hESC research holds huge promise for treating major human diseases that have been challenging for traditional medicine, such as a wide range of incurable or hitherto untreatable neurological and heart diseases. Millions of people are pinning their hopes on hESC research. As neurological and cardiovascular diseases incur exorbitant costs on the healthcare system worldwide, there is a strong focus on translating hESC research innovations to provide potentially life-saving treatments or cures for these major health problems. However, a persistent challenge for clinical translation of hESC research is how to turn non-functional pluripotent cells into the precise functional somatic cell type we need for repair.
Xcelthera has developed the key breakthrough PluriXcel Technology Platforms to solve this problem, enabling well-controlled, highly efficient, direct conversion of non-functional clinical-grade hESC at the pluripotent stage by small molecule induction into a large supply of functional human neuronal/cardiac progenitor/precursor cells (Xcel prototypes) as novel, effective regenerative medicine advanced therapy (RMAT) products for neuron-circuitry/heart-muscle regeneration, overcoming the major bottlenecks in the regenerative medicine market (USPTO patent# 9,428,731 and 8,716,017). PluriXcel Technology Platforms are game-changing enabling technologies to provide RMAT products in large quantity and high quality with adequate cellular capacity to regenerate the neuron circuitry and the contractile heart muscle, ensuring high degrees of efficacy and safety of the hESC-derived therapeutic products, thus robust clinical benefit leading to therapies. It not only constitutes clinically representative progresses in both human neuronal and cardiac therapeutic products for treating a wide range of incurable or hitherto untreatable neurological and cardiovascular diseases, but also offers manufacturing innovation for production scale-up and creation of replacement tissue or organ products. Our breakthrough innovations present hESC as a novel, advanced therapeutic strategy for a wide range of incurable or hitherto untreatable neurological and heart diseases, having tremendous impact on economy, health, future medicine, and patient care.
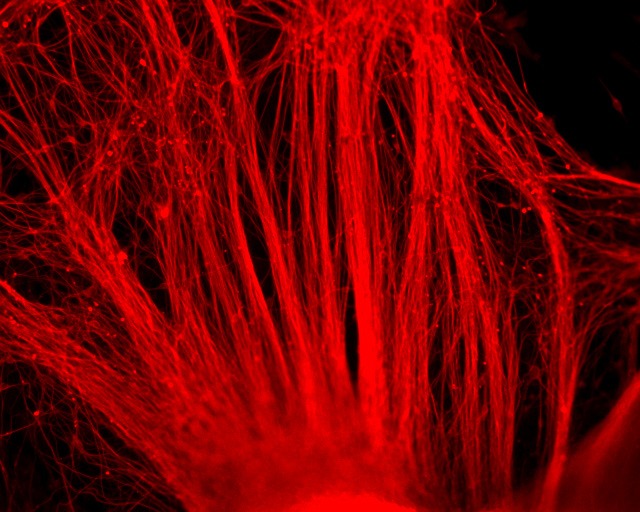
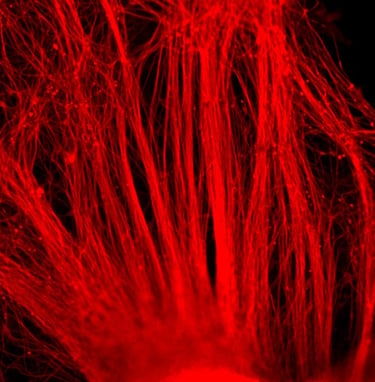
Company Overview
Xcelthera uniquely holds the key breakthrough technologies and exclusive rights for the major bottlenecks in the regenerative medicine market. As a major innovator in stem cell research, Xcelthera has secured robust IP position in the global regenerative medicine market with issued patents and exclusive rights for development and commercialization of relevant hESC-based cell therapy products and tools.
PluriXcel human stem cell technology platforms, including PluriXcel-DCS (defined culture system), PluriXcel-SMI (small molecule induction)-Heart, PluriXcel-SMI-Neuron, provide proprietary clinical translating capabilities to address key challenges to traditional medicine and biofabrication. Its hESC-based Xcel cell therapy products, including Xcel-hCardP, Xcel-hCM, Xcel-hNuP, Xcel-hNu, offer currently the only available human cell sources in large quantity and high quality with adequate cellular capacity to regenerate the contractile heart muscle and the neuron circuitry, overcoming major bottlenecks for tissue repair and biofabrication. Our overall strategy is to leverage hESC-based stem cell therapy and regenerative medicine development and manufacturing innovations to provide the next generation of cell-based therapeutic solutions for unmet medical needs in world-wide major health problems, including scale-up production of clinical-grade hESC-derived human heart and neuronal stem/progenitor/precursor cells, and functional human heart muscle and neuronal cell/tissue products supplied to clinical trials; clinical trials of human heart precursor cells (Xcel-hCardP) and heart muscle cells (Xcel-hCM) for contractile heart muscle regeneration or replacement therapies (product pipeline includes myocardial infarction, cardiomyopathy, heart failure); clinical trials of human neuronal progenitor cells (Xcel-hNuP) and neurons (Xcel-hNu) for neuron regeneration or replacement therapies (product pipeline includes Parkinson’s disease, spinal cord injury, stroke, traumatic brain injury, spinal muscular atrophy); developing replacement 3D heart tissue products and hearts for heart transplantation therapies (solving the problem of the acute shortage of donor organs); developing replacement 3D neuronal tissue products and micro-brains/spinal-cords for brain and spinal cord transplantation therapies. Xcelthera has demonstrated the safety and efficacy of its products for heart and neurological indications/conditions and human stem cell production at scale for entry into clinical trials.
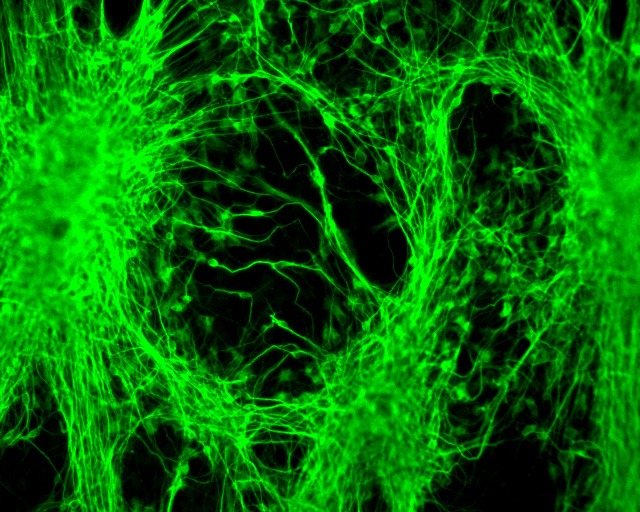
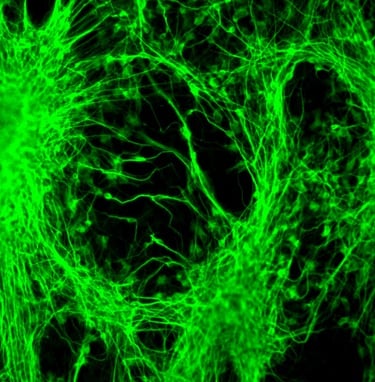
Product Overview
Xcelthera is the only company with regenerative medicine advanced therapy (RMAT) products that have demonstrated production at scale and product purity/potency for heart and neurological tissue and function restoration in the clinical setting, thus a high probability leading to treatments or cures for a wide range of incurable or hitherto untreatable neurological and heart diseases, including heart disease and failure, stroke, Parkinson’s disease (PD), Alzheimer disease (AD), spinal cord injury (SCI), traumatic brain injury (TBI), amyotrophic lateral sclerosis (ALS), spinal muscular atrophy (SMA). Xcelthera’s hESC-based RMAT products represent the next generation of human cell therapy products, offering purity, large-scale production, high quality, safety, and effectiveness for commercial and therapeutic uses over all other existing human cell sources or products. Our lead hESC-based RMAT products include clinical-grade high purity human heart precursor cells (Xcel-hCardP) and heart muscle cells (Xcel-hCM) for contractile heart muscle regeneration or replacement therapies, as well as clinical-grade high purity human neuronal progenitor cells (Xcel-hNuP) and neurons (Xcel-hNu) for neuron regeneration or replacement therapies.
Competitive Advantages
Traditional drug development usually starts with drug leads discovered in non-human simple model organisms, thus requires lengthy and costly both demonstration in animal model testing and establishment of proof-of-concept and safety in human trials. As a result, millions of drug leads have vanished before even reach clinical trials, and for few lucky ones, have encountered the very high drug failure rate in human trials. Among those very few drugs that eventually obtained their market approvals, there were not any cures, or even meaningfully effective treatments, for Alzheimer disease, Parkinson’s disease, stroke, spinal cord and brain injuries, heart disease and failure, or a host of other disorders that destroy lives.
Unlike traditional R&D, hESC-based RMAT products have been developed directly with human cells with proof-of-concept already established in humans, which simplifies the development process, lowers the costs, shortens the time consumption, and increases the probability of clinical success dramatically. PluriXcel technology platforms are designed and developed specifically to address practical issues essential for designating any human stem cell as a safe and effective cell therapy product for filing IND and entry into human trials, including the benefits in high efficiency, stability, low tumor risk, high purity, high efficacy in repair, safety, as well as stem cell production at large scale, over all other existing stem cell sources or products.
Patents and exclusive rights have been secured to access the key translating technologies and capabilities in the regenerative medicine market, which allow derive and establish therapeutically viable and clinically useful high quality hESC-derived human cell therapy products/tools/banks, design and execute a cost-effective cell therapy product development and manufacturing strategy, and implement cGMP-compatible/quality manufacturing processes for production scale-up and creation of replacement cell/tissue/organ products.
PluriXcel platforms focus on developing regenerative medicine advanced therapies (RMAT) targeting serious or life-threatening diseases and conditions in unmet medical needs that have been big challenges for traditional medicine. Therefore, Xcelthera’s hESC-based RMAT products will not only significantly increase the success rate in clinical trials and reduce the costs of registration pipeline as they reach cGMP-compliance and subsequent clinical phases, but also qualify for FDA expedited regulatory programs, including Fast-Track Program, Breakthrough Therapy Designation Program, priority review, and the Regenerative Medicine Advanced Therapy (RMAT) Designation Program to accelerate regulatory review/approval and patient access to new therapies brought by the breakthrough medical innovations of hESC research.